Decomposition of Zinc Nitrate
Photo decomposition occurs when a complex chemical is exposed to photons from light. The nitrate ions react with blood hemoglobin.

The Role Of Anhydrous Zinc Nitrate In The Thermal Decomposition Of The Zinc Hydroxy Nitrates Zn5 Oh 8 No3 2 2h2o And Znohno3 H2o Sciencedirect
Try this demonstration to create a mini volcanic eruption illustrating the decomposition of ammonium dichromate.
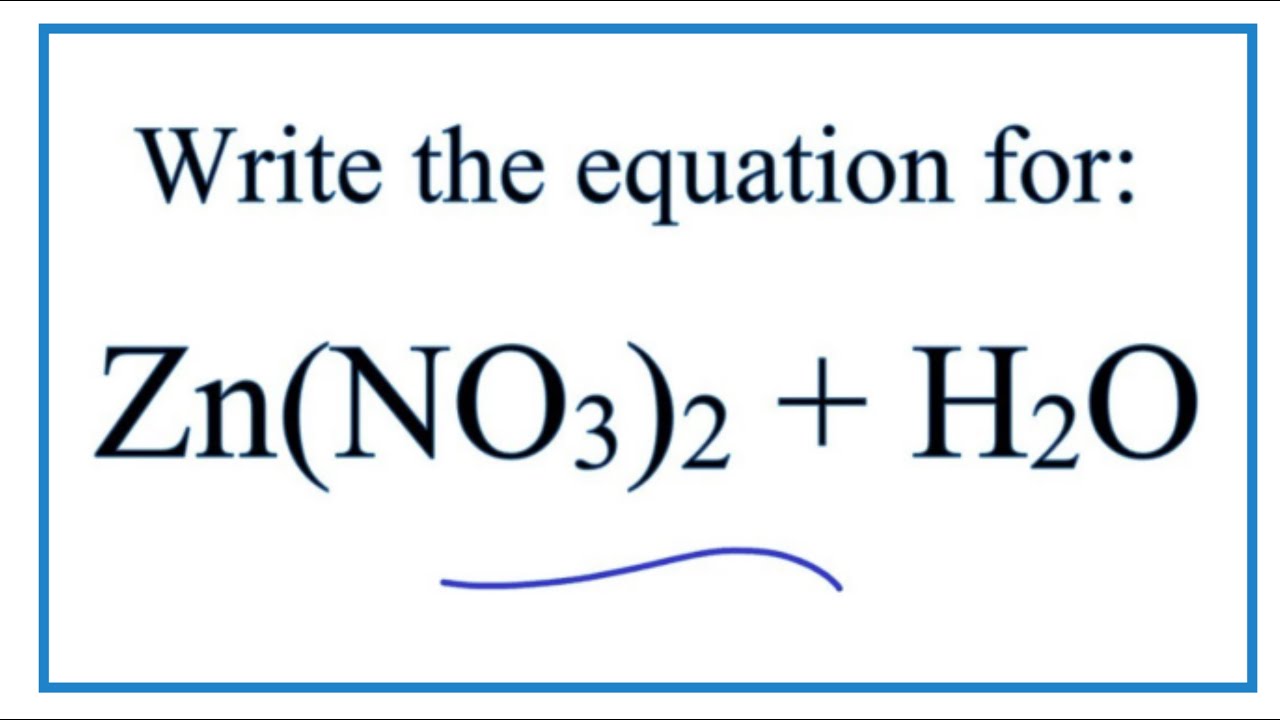
. Zinc can be removed by dummying the bath at 0204 Adm 2. Zinc has many commercial uses as coatings to prevent rust in dry cell batteries and mixed with other metals to make alloys like brass and bronze. 3 Use Asbestos Limit 19261101.
Decomposition reactions can be classified as either exothermic or endothermic depending on whether they release or absorb. Contrairement à dautres sels de plombII il est soluble dans leauSon usage principal depuis le Moyen Âge sous le nom de plumb dulcis a été comme matière première de nombreux pigmentsDepuis le XX e siècle il est utilisé comme. Thermal decomposition breaks the chemical down with heat while electrolytic decomposition uses electricity to break the bonds.
Dummying also removes many other metallic contaminants. Il sagit aussi de lancien nitrate dammoniaque obtenu industriellement depuis le XX e siècle par un mélange dammoniaque et dacide nitrique. A hierarchically three-dimensionally ordered mesoporous 3DOM Mg x Co 3x O 4 is engineered as an efficient and durable bifunctional catalyst for both ORR and OER.
Sam and Sally collected 085 g of zinc chloride. Chemical Product and Company Identification. Copper Cu and zinc Zn are nontoxic if found in small concentrations.
However NFPA 490 remains relevant as the basis for 1910109i. A zinc and copper alloy is used to make pennies in the United States. If the decomposition of 200 g of KCIO_3 gives 0720 g of O_2 what is the percent yield for the reaction.
Chloride with metallic zinc in class. The bacterial reduction of nitrate can also produce nitrite under anaerobic conditions. 2 Remotely actuated mechanical ventilation is preferred for fire response.
It is found in air soil and water and is present in all foodsPure zinc is a bluish-white shiny metal. In a double replacement reaction between silver nitrate and magnesium bromide. Mole ratios are used as conversion factors between products and reactants in stoichiometry calculations.
5 See Table 2 of this section for the exposure limit for any operations or sectors where the exposure limit in 19261153 is stayed or is otherwise not in effect. Nitrite nitrogen occurs as an intermediate stage in the biological decomposition of ammoniaammonium. Can be neglected at ambient conditions.
Zinc carbonate is first decomposed to give zinc oxide and then reduced to obtain zinc metal. Le nitrate de plombII est un sel inorganique de plomb et dacide nitriqueCest un cristal incolore ou une poudre blanche et un oxydant stable et fort. Lead Nitrate Decomposition Reaction.
1 NFPA withdrew NFPA 490 following the 2002 edition and incorporated requirements for storage of ammonium nitrate into NFPA 400 Chapter 11. Footnotes to Tables 1 and 2 of this section. The presence of nitrate increases.
A decomposition reaction is a type of reaction where bonds are broken and new ones form. How many moles of O₂ are required to form 500 moles of H₂O. 2 See Table 2 of this section.
A solidsolid reaction between lead nitrate and potassium iodide. Includes kit list and safety instructions. Reactions of chlorine.
For example in the reaction. Includes kit list and safety instructions. Compare the rate of reaction between zinc and sulfuric acid with.
380C 716F Melting Point. 3210 Copper and zinc. An X designation in the Skin Designation column indicates that the substance is a.
The main source of nitrogen compounds in water are fertilizers that mainly contain nitrate but also ammonia ammonium urea and amines. 2H₂g O₂g 2H₂Og The mole ratio between O₂ and H₂O is 1 mol O₂2 mol H₂O. This is a result of the decomposition of free cyanide through hydrolysis and cyanide oxidation at the anode.
An equilibrium using copperII and ammonia. Try this demonstration to create a mini volcanic eruption illustrating the decomposition of ammonium dichromate. By virtue of the unique architecture and effective modulation of chemical environment of Co and Mg atoms the zinc-air battery assembled with 3DOM Mg x Co 3x O 4 exhibits a high power density of 253 mW cm 2.
Ammonium nitrate --- water and dinitrogen oxide laughing gas There are three ways to break down chemicals into simpler substances. Le nitrate dammonium est un composé ionique du cation ammonium et de lanion nitrate de formule N H 4 N O 3Il correspond au corps minéral anhydre naturel de maille orthorhombique nommé par les minéralogistes nitrammite 8. It is prepared by the action of zinc on dilute nitric acid by the action of hydroxylamine hydrochloride NH 2 OHHCl on sodium nitrite NaNO 2 and most commonly by the decomposition of ammonium nitrate NH 4 NO 3.
3 The IDLH concentration of nitrogen dioxide one of the principal. Thus the chemical decomposition 42 of unstable H 2 N 2 O 2 to N 2 O is considered 8898. Even though zinc recommended MCL following secondary drinking water standards by EPA are as high as 50 mg L 1.
Dinitrogen pentoxide is the chemical compound with the formula N 2 O 5 also known as nitrogen pentoxide or nitric anhydrideIt is one of the binary nitrogen oxides a family of compounds that only contain nitrogen and oxygenIt exists as colourless crystals that melt at 41 C. The complete decomposition of organic material by microorganisms takes time usually 20 d or more under ordinary circumstances. Page 1 of 1 MSDS - Sodium Nitrate Material Safety Data Sheet MSDS - Sodium Nitrate 1.
Zinc is one of the most common elements in the earths crust. The Editors of Encyclopaedia Britannica This article was most recently revised and updated by Adam Augustyn. As zinc or aluminum or aluminum oxide isothiocyanates thiocyanates phosphorus.
After fertilization crops take up a relatively small part of added nitrogen compounds. Its boiling point is 47 C and sublimes slightly above room temperature yielding a colorless gas. Includes kit list and safety instructions.
Autotrophic bacteria convert ammonia under oxic aerobic conditions to nitrates. Oxide and zinc in this class demonstration. Critical review on fundamentals and challenges of nitrate electrocatalytic reduction.
The most widely applied nitrogen fertilizers are probably NaNO 3 sodium nitrate and NH 4 NO 3 ammonium nitrate. A solidsolid reaction between lead nitrate and potassium iodide. However carbonate forms naturally during operation in all cyanide baths.
The mole ratio between H₂ and H₂O is 2 mol H₂2 mol H₂O.

How To Balance Zn No3 2 Zno No2 O2 Zinc Nitrate Decomposition Youtube
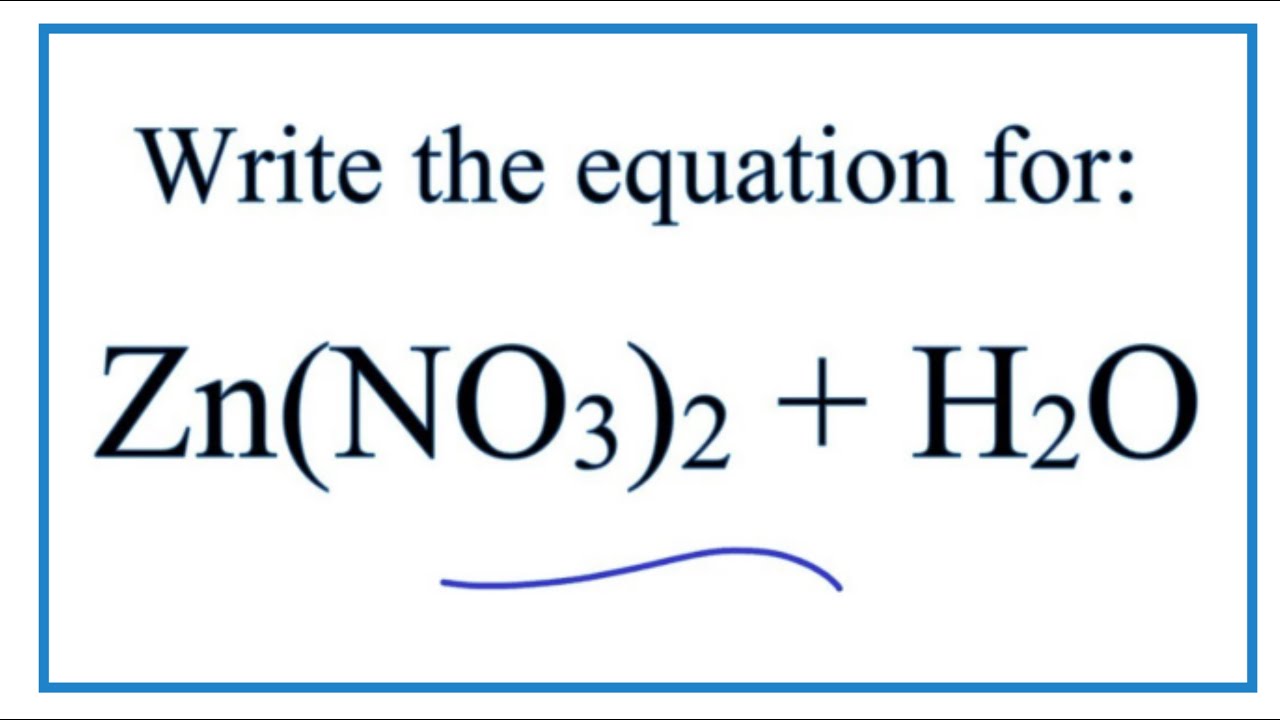
Equation For Zn No3 2 H2o Zinc Nitrate Water Youtube

The Formula Of Zinc Nitrate Is

The Action Of Heat On Zinc Ii Nitrate Laboratory Experiments On Salts Youtube
No comments for "Decomposition of Zinc Nitrate"
Post a Comment